Radium
88
Ra
Skupina
2
Perioda
7
Blok
s
Protony
Elektrony
Neutrony
88
88
138
Hlavní vlastnosti
Atomové číslo
88
Atomová hmotnost
[226]
Hmotnostní číslo
226
Kategorie
Kovy alkalických zemin
Barva
Stříbrná
Radioaktivní
Ano
Z latinského slova radius znamenající paprsek
Krystalografická soustava
Kubická prostorově centrovaná
Historie
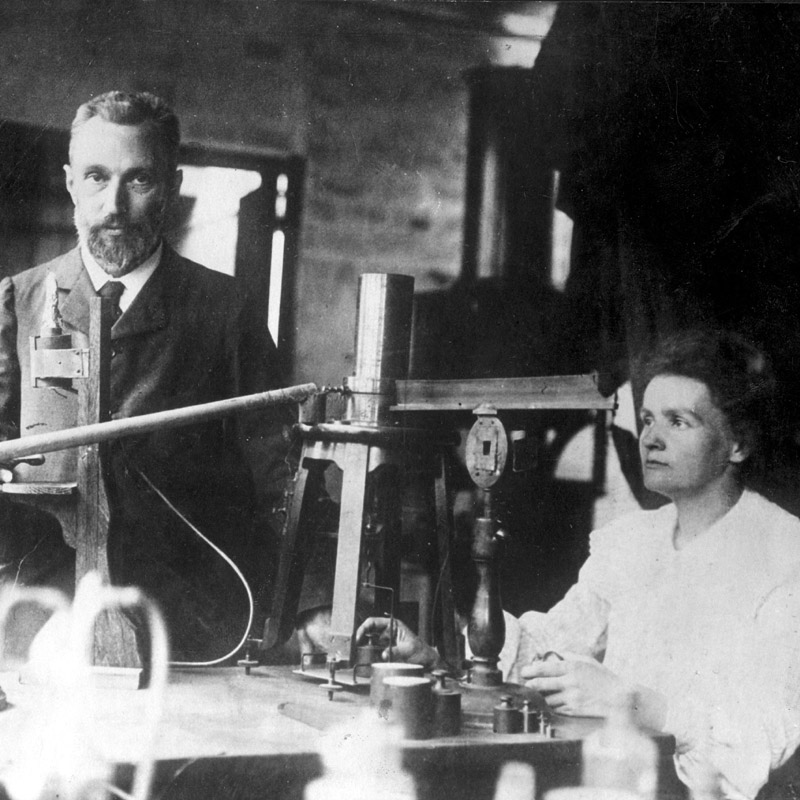
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Elektronů v obalu
2, 8, 18, 32, 18, 8, 2
Elektronová konfigurace
[Rn] 7s2
Radium imparts a carmine red color to a flame
Fyzické vlastnosti
Skupenství
Pevná
Hustota
5,5 g/cm3
Teplota tání
973,15 K | 700 °C | 1292 °F
Teplota varu
2010,15 K | 1737 °C | 3158,6 °F
Skupenské teplo tání
8 kJ/mol
Skupenské teplo varu
125 kJ/mol
Měrná tepelná kapacita
- J/g·K
Hojnost v zemské kůře
9,9×10-12%
Hojnost ve vesmíru
n/a
Číslo CAS
7440-14-4
PubChem CID číslo
6328144
Atomové vlastnosti
Atomový poloměr
-
Kovalentní poloměr
221 pm
Elektronegativita
0,9 (Paulingova stupnice)
Ionizační potenciál
5,2784 eV
Molární objem
45,20 cm3/mol
Tepelná vodivost
0,186 W/cm·K
Oxidační čísla
2
Aplikace
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium je vysoce radioaktivní karcinogenní
Izotopy
Stabilní izotopy
-Nestabilní izotopy
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra